Introduction
Taxonomy and Classification of the Family Scytosiphonaceae
Systematic Revisions in the Scytosiphonaceae: The way Forward
Introduction
The brown algal family Scytosiphonaceae Ardissone and Strafforello (1877) (Ectocarpales, Phaeophyceae) is an interesting group of benthic marine macroalgae that inhabit the coasts and offshore reefs of tropical to warm temperate regions of the Atlantic, Indian, Pacific, and Arctic Oceans. Members of the family Scytosiphonaceae are characterized by possessing a single cup-shaped plastid bearing a large pyrenoid and exhibiting a heteromorphic life history (except some taxa) where an erect, macroscopic gametophyte alternates with a prostrate, microscopic sporophyte (Nakamura and Tatewaki 1975, Kogame et al. 1999, Toste et al. 2003, Silberfeld et al. 2011, Santiañez et al. 2018a).
The family Scytosiphonaceae has been subjected to several systematic revisions in recent decades, owing largely to the information derived from molecular-based taxonomic studies. The Scytosiphonaceae was initially classified under the order Scytosiphonales Feldmann (1949), along with the family Chnoosporaceae. The two families were segregated primarily by the difference in their growth patterns (i.e., diffuse in the Scytosiphonaceae and sub-apical in the Chnoosporaceae) (Setchell and Gardner 1925, Feldmann 1949, Kogame 2001, Cho et al. 2006). Molecular phylogenetic studies, however, suggested that the Scytosiphonales and the Chnoosporaceae are artificial groups. That is, Rousseau and Reviers (1999) proposed that the Scytosiphonales be reduced to familial rank (Scytosiphonaceae) and be classified in the Ectocarpales sensu lato, along with other brown algal orders with single prominent pyrenoids [i.e., Orders Chordariales Setchell and Gardner (1925), Dictyosiphonales Setchell and Gardner (1925)]. Within the Scytosiphonaceae, the pioneering molecular phylogenetic studies on the family by Kogame et al. (1999) revealed problematic relationships among scytosiphonacean taxa, highlighting the inconsistencies between their taxonomies that are largely based on the gross morphologies of the erect and macroscopic gametophytes and their phylogenetic positions and relationships. In the same work, Kogame et al. (1999) proposed delineating generausing morphologies of microscopic sporophytes as these correspond well with the topology of their molecular phylogenetic trees. In a subsequent study by Cho et al. (2003) that included Myelophycus Kjellman in Engler & Prantl, a genus that possesses an isomorphic life history, emended the description of the Scytosiphonaceae to include this life history characteristics within the family. Prior to this work, the classification of Myelophycus had remained questionable as the genus was placed either in the Punctariaceae or in the Chordariaceae (Tanaka and Chihara 1984). Subsequent phylogenetic studies on the Scytosiphonaceae by Lee et al. (2014a) including the genus Melanosiphon M.J.Wynne (1969), a brown seaweed genus that also exhibits an isomorphic life history, provided molecular evidence to the inclusion of the genus within the Scytosiphonaceae. To date, the family Scytosiphonaceae includes 15 genera: Chnoospora J.Agardh (Fig. 1A), Colpomenia (Endlicher) Derbès & Solier (Fig. 1B), Dactylosiphon Santiañez, K.M.Lee, S.M.Boo & Kogame, Encephalophycus Santiañez, Hapterophycus Setchell & N.L.Gardner, Hydroclathrus Bory de Saint-Vincent (Fig. 1C), Iyengaria Børgesen (Fig. 1D), Jolyna S.M.Guimarães (Fig. 1E), Manzaea Santiañez & Kogame (Fig. 1F), Melanosiphon M.J.Wynne (Fig. 1G), Myelophycus Kjellman (Fig. 1H), Petalonia Derbès & Solier (Fig. 1I), Planosiphon McDevit & G.W.Saunders, Pseudochnoospora Santiañez, G.Y.Cho & Kogame (Fig. 1J), Rosenvingea Børgesen, Scytosiphon C.Agardh (Fig. 1K), and Tronoella Santiañez & Kogame (Fig. 1L). The genus Symphyocarpus Rosenvinge is also currently assigned to the family Scytosiphonaceae, but this classification is yet to be confirmed by molecular data.

Fig. 1.
Some representative members of the family Scytosiphonaceae. A, Holotype specimen of the upright and branching Chnoospora minima (K. Hering) Papenfuss (HBG 024509). B, Possible (iso)type specimen of Colpomenia sinuosa (Mertens ex Roth) Derbès & Solier (A 37119) deposited at the Sweden Museum of Natural History, Stockholm (S). C, Holotype specimen of the net-like and amorphous Hydroclathrus clathratus (C. Agardh) Howe (BM 000563484). D, Branching and hollow Iyengaria stellata (Børgesen) Børgesen collected from the type locality in Dwarka, India (MICH 636037). E, Upright, branching, and flattened thallus of Jolyna furcata (MICH 636068) currently endemic in the Sultanate of Oman. F, Net-like, amorphous, and inter-adhesive thallus of the holotype specimen of Manzaea minuta (Santiañez & Kogame) Santiañez & Kogame (SAP 115290). G, Possible holotype specimen of Melanosiphon intestinalis (D.A. Saunders) M.J.Wynne (NY 02257508) deposited at the Herbarium of the New York Botanical Garden. H, Erect and unbranched thallus of Myelophycus caespitosus Kjellman (NY 0225770) collected from Shima, Japan. I, Erect, leaf-like, and flattened thallus of Petalonia fascia (O.F.Müller) Kuntze (SAP 050352). J, Holotype specimen of the branching and inter-adhesive Pseudochnoospora implexa (J. Agardh) Santiañez, G.Y. Cho & Kogame (BM 000569565). K, Erect, constricted, and hollow thallus of Scytosiphon lomentaria (Lyngbye) Link (SAP 059358). L, Holotype specimen of the Tronoella ryukyuana Santiañez & Kogame (SAP 115297) currently endemic to Okinawa, Japan. Scale bars = 2 cm.
The splitting of several genera in the Scytosiphonaceae to address the extensive paraphyly and polyphyly observed in the molecular phylogeny of the family—facilitated through the works of McDevit and Saunders (2017), Santiañez et al. (2018a, 2018b), Santiañez and Kogame (2022), and Santiañez (2022)—considerably increased the genus-level diversity in the Scytosiphonaceae (Fig. 2, Table 1). In their efforts to resolve the molecular phylogenetic relationships within Scytosiphon, McDevit and Saunders (2017) considered pooling several species into a single, widely circumscribed genus or to recognize and clearly delineate the morpho-anatomical boundaries of a new taxonomic group based on the principle of monophyly (Santiañez 2018). In their systematic treatment, McDevit and Saunders (2017) rejected the first option as it would mean merging several taxa with distinct morpho-anatomies and life histories into one genus and is therefore problematic. Following the framework outlined by McDevit and Saunders (2017), subsequent works on the systematics of the Scytosiphonaceae where molecular information was integrated with morphology, anatomy, ecology, and life history [e.g., Santiañez et al. (2018a, 2018b), Santiañez and Kogame (2022), and Santiañez (2022, 2023)], have since resulted in significant changes in our understanding of the diversity and phylogenetic relationships within the family (Fig. 2, Table 1). Despite these efforts, the taxonomy, classification, and phylogenetic relationships of some scytosiphonacean taxa such as Colpomenia and Rosenvingea have remained confused.
Herein, we provide an overview of the systematics of the family Scytosiphonaceae in the advent of molecular phylogenetic studies. Highlighted herein are the challenges in understanding the diversity and systematics of the family and the framework for resolving the complex taxonomy, classification, and nomenclature of scytosiphonacean taxa across several ocean basins.
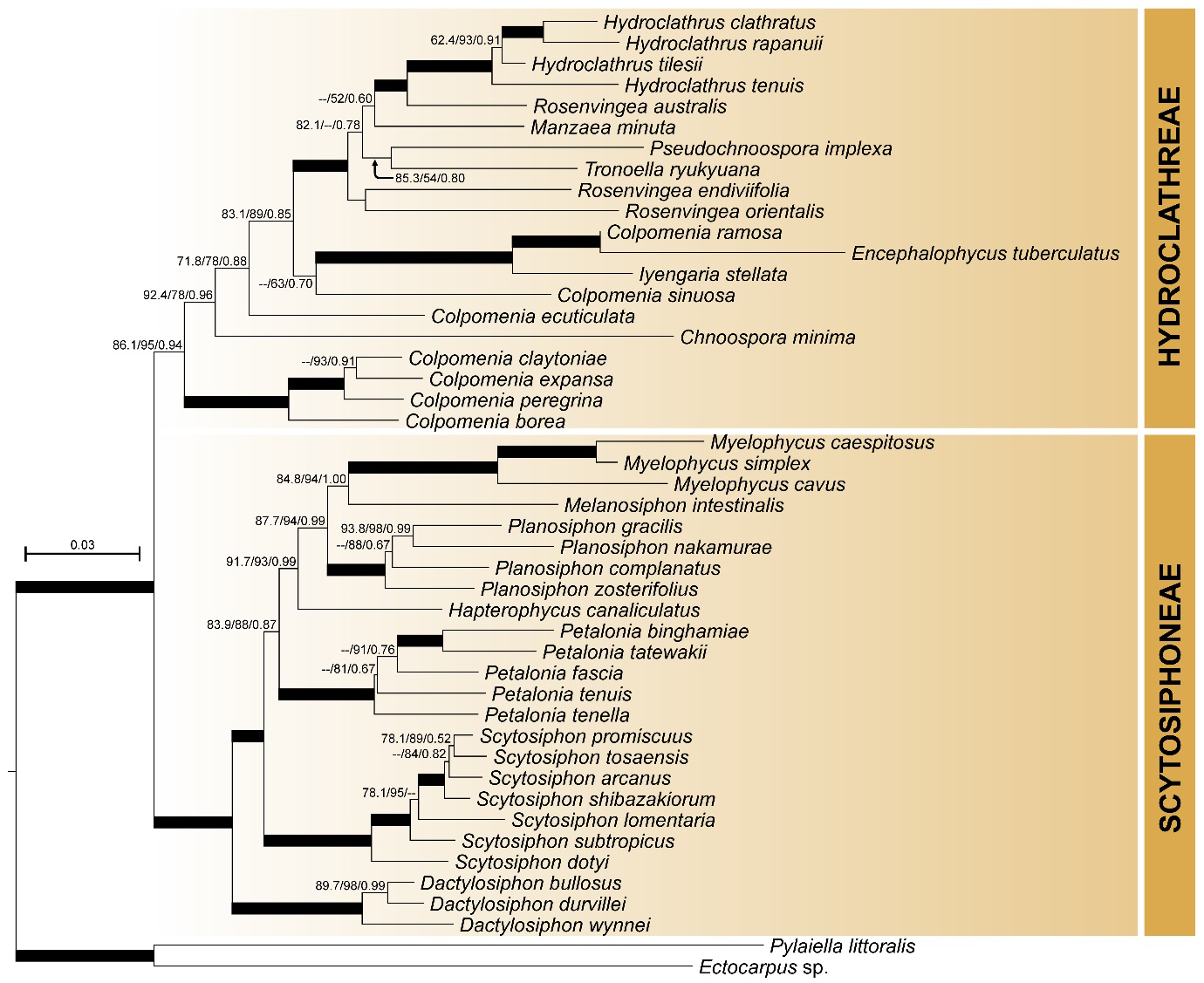
Fig. 2.
Maximum likelihood tree of family Scytosiphonaceae inferred from concatenated sequence data (cox3+cox1+rbcL+psaA+psbA). Support values in the form of approximate likelihood ratio test percentages (aLRT), ultrafast bootstrap (UFBS) and Bayesian posterior probabilities (BPP) are shown at each branch. Thickened branches indicate highly supported nodes (aLRT: >95; UFBS: >95; BPP: >0.95). Values less below the threshold (aLRT: <50; UFBS: <50; BPP <0.50) are not shown. Molecular phylogenetic analyses based on concatenated gene trees followed those of Santiañez et al. (2018b, 2023).
Table 1.
Classification of taxa within the family Scytosiphonaceae
Taxonomy and Classification of the Family Scytosiphonaceae
The problems in the taxonomy, classification, and molecular phylogenetic relationships in the Scytosiphonaceae are primarily due to the broad and ambiguous morphology-based delineation of each genus (Kogame et al. 1999, Santiañez et al. 2018a, 2018b, Santiañez and Kogame 2022). This challenge is also further compounded by the highly plastic morphologies of the different species, consequently blurring genus-level boundaries. We provide below how these problematic taxonomic and classification schemes of scytosiphonacean taxa at the genus and species levels are clarified with the help of molecular phylogenetic data integrated with the known life history characteristics of several representative taxa.
The erect to decumbent species of Chnoospora are characterized by having branched thalli that are free or inter-adhesive. The gross morphology of Chnoospora species is similar to the closely related and similarly branched Rosenvingea, but the former are distinguished by having a solid thallus, while the latter possess a hollow, cylindrical thallus. Previously, all known Chnoospora species were classified in the monogeneric family Chnoosporaceae due to their subapical growth pattern (Setchell and Gardner 1925). Molecular evidence to abandon the Chnoosporaceae and classify Chnoospora species under Scytosiphonaceae was provided by Kogame et al. (1999), based on molecular phylogenetic information derived from a specimen of Chnoospora implexa J.Agardh. In the subsequent molecular-based study on the Scytosiphonaceae by Cho et al. (2006) including Chnoospora minima (K.Hering) Papenfuss, they showed that Chnoospora is a polyphyletic genus. Noting that C. minima has been designated as the lectotype of the genus Chnoospora (Huisman 2015) and that J.Agardh (1848) placed C. implexa (as Sphaerococcus implexus Hering nom. nud. under ‘Species inquirenda’, Santiañez et al. (2018b) questioned the generic assignment of C. implexa. Santiañez et al. (2018b) argued that C. implexa presents several characteristics that are very different from C. minima including the decumbent thalli that are attached via rhizoidal holdfasts when in contact with the substrates, inter-adhesive branches, and the solid thallus construction [vs partially hollow in C. minima (Santiañez et al. (2018a, 2018b)]. By integrating this morpho-anatomical information with that derived from their molecular phylogeny, Santiañez et al. (2018b) transferred C. implexa to their newly described genus Pseudochnoospora Santiañez, G.Y.Cho & Kogame. To date, the genus is considered to be monotypic and represented only by its type species Pseudochnoospora implexa (J.Agardh) Santiañez, G.Y.Cho & Kogame (Table 1).
The widely distributed genus Colpomenia is considered as the most variable in the Scytosiphonaceae, with members possessing saccate, irregularly convoluted, and branching thalli. As such, Colpomenia species have morphologies that are known to overlap with species from the genus Iyengaria (Wynne 1972), Rosenvingea, and Scytosiphon (Wynne and Norris 1976), resulting in the confused classification of several species. Studies on the family Scytosiphonaceae by Kogame et al. (1999) and Cho et al. (2003, 2006) have repeatedly shown the unresolved relationships among Colpomenia species, somewhat echoing the ongoing debate on how to delineate morpho-species boundaries among Colpomenia species (e.g., Clayton 1975, Wynne and Norris 1976, Parsons 1982). Colpomenia has a long and problematic taxonomic history, which primarily stems from the ambiguously wide circumscription of the genus. Colpomenia traditionally includes brown seaweeds with saccate, upright and/or branched thalli that are hollow and thin-walled. Typically, Colpomenia species also produce ascocysts along with their plurangia, which may or may not have a cuticle cover. Sporophytic thalli of Colpomenia are also known to have variable characteristics, including producing unangia only or both unangia and plurangia on the same thallus (Womersley 1987, Kogame 1997, Boo et al. 2011, Lee et al. 2012, 2013, Kraft 2009). Previous studies have attempted to resolve some of the species-level taxonomic problems within Colpomenia including studies by Vandermeulen et al. (1984) for Co. sinuosa (the generitype), Co. peregrina, and Co. bullosa. In their work, Vandermeulen et al. (1984) selected a neotype for Co. sinuosa based on a BM (The Natural History Museum, London) specimen collected from Tenerife, Spain, as the type specimen collected by Mertens from Cádiz, Spain (type locality), is presumed lost, and efforts to locate it have proven to be futile. The neotype selected by Vandermeulen et al. (1984) has the typical cuticulate plurangial sori, but the shape of its thallus was unlike those illustrated by Roth (1806, pl. 12). Among the possible herbaria that is presumed to house Mertens’ specimen is the Sweden Museum of Natural History, Stockholm (S). An on-line search of ‘Sweden’s Virtual Herbarium’ (http://herbarium.emg.umu.se/standard_search.html) resulted in finding a Co. sinuosa specimen (code A37119) collected from Cádiz by Mertens. This C. sinuosa A37119 specimen, with specimen label containing “Aspercoccus sinuosus Bory. / Ulva sinuosa m n. descr. / in 3fas Catal. Fasc. Icon […] / Prope Gades. / Mertens scripsit et misit. Herb. Swartzii”, closely resembles the shape of the epiphytic Colpomenia illustrated by Roth. As such, we opined that neotypification of Co. sinuosa should be based on the specimen from S rather than those assigned by Vandermeulen et al. that is currently deposited in BM. Considering the issues in identifying Colpomenia species, detailed taxonomic studies must first be done on A37119 to confirm its identity.
Molecular-assisted taxonomic studies on saccate and elongate Colpomenia have resulted in descriptions of new species and discovery of a species complex within Co. sinuosa (Boo et al. 2011, Lee et al. 2012, 2013, 2014b), but the problematic relationship between the saccate and elongate species remained unresolved. To address this problem, Santiañez et al. (2018b) used concatenated molecular data in reconstructing the molecular phylogeny of the Scytosiphonaceae. Noting the differences in their morphologies (saccate vs elongate), life history characteristics (presence of unangia only vs presence of both unangia and plurangia), and their phylogenetic segregation, Santiañez et al. (2018b) established the new genus Dactylosiphon to accommodate the elongate species of Colpomenia. More recently, Santiañez (2022) reassessed the taxonomy and phylogenetic position of Colpomenia tuberculata D.A.Saunders, a tuberculate and convoluted species originally described from near San Pedro, California (Saunders 1898). Based on comparisons of known morpho-anatomical characteristics of Co. sinuosa and Co. tuberculata, Santiañez (2022) outlined significant differences in the shape and nature of its thallus, cortical and medullary cells, as well as the sequence divergences between the two species. Integrating information derived from his rbcL-based molecular phylogenetic studies and the morpho-anatomical differences of Co. tuberculata, Santiañez (2022) segregated the species and established the new genus Encephalophycus. Currently, Colpomenia has eight species, but the branching morphology and segregation of Colpomenia ramosa W.R.Taylor and its close affinity with Encephalophycus tuberculatus (D.A.Saunders) Santiañez based on cox3 gene-based trees (e.g., Santiañez et al. 2018a, 2018b) suggest the need to reassess its taxonomy and classification. In cox3 and rbcL-gene phylogenetic trees (e.g., Santiañez et al. 2018a, 2018b, Santiañez 2022), Colpomenia claytoniae S.M.Boo, K.M.Lee, G.Y.Cho & W.Nelson, Colpomenia expansa (D.A.Saunders) Y.-P.Lee, and Colpomenia peregrina Sauvageau, are often recovered as a monophyletic clade that is segregated from other saccate species including the generitype Co. sinuosa. Similarly, the branched and inter-adhesive Colpomenia ramosa W.R.Taylor has been repeatedly shown to be segregated from Co. sinuosa and clustering with E. tuberculatus in cox3 gene-based trees (e.g., Lee et al. 2014a, 2014b, Santiañez et al. 2018b). Our multigene tree also recovered Co. ramosa in between I. stellata and E. tuberculatus, with very strong support. The unique morpho-anatomy and phylogenetic position of Co. ramosa suggest an erroneous genus assignment. Whether these taxa should be classified under Colpomenia or should be transferred to a different genus would need detailed studies on their morpho-anatomies and life history characteristics. Nevertheless, Colpomenia, as currently circumscribed, is represented by nine species (Table 1).
Hydroclathrus species are among the most easily identifiable brown seaweeds in tropical to subtropical waters, owing to their distinct net-like and spreading thalli (Santiañez et al. 2018a). Young Hydroclathrus thalli are saccate and can be mistaken with Co. sinuosa. In fact, prior to the description of the genus Colpomenia, Co. sinuosa was considered as a species of Hydroclathrus by Zanardini (1843) (as Hydroclathrus sinuosus (Mertens ex Roth) Zanardini). Indeed, the genus-level distinction of Colpomenia and Hydroclathrus has long been debated (Wynne 1972).
The first detailed assessment of the taxonomy and some aspects of the ecology of the genus Hydroclathrus was done by Kraft and Abbott (2003) based on global samples. Therein, they outlined the distinct genus-level delineation of Hydroclathrus but also highlighted the blurred distinction between the two common species, the generitype Hydroclathrus clathratus (C.Agardh) M.Howe and Hydroclathrus tenuis C.K.Tseng & Lu Baoren. This problem is also aggravated by the different interpretations of various taxonomists as to what is H. clathratus and what is H. tenuis. Kraft and Abbott (2003) therefore advocated the use of DNA phylogenies to resolve the problem. Nonetheless, Kraft and Abbott (2003) added two new species to the genus (Hydroclathrus stephanosorus Kraft and Hydroclathrus tumulis Kraft & Abbott) based on morpho-anatomical observations and ecological information.
Santiañez et al. (2018a) were the first to address the problem on the identities of Hydroclathrus species by conducting molecular-assisted taxonomic studies on Hydroclathrus collected from the Pacific, Indian, and Atlantic Oceans. Their work confirmed that H. stephanosorus is a distinct taxon that is widely distributed in the Indo-Pacific and in the eastern Atlantic Oceans. Additionally, they also highlighted the very high plasticity of H. tenuis as they can also grow with relatively broad membrane and robust thalli, typical to H. clathratus. In the same work, Santiañez et al. (2018a) described a new warm-water species H. minutus Santiañez & Kogame as well as the new genus and species, Tronoella ryukyuana Santiañez & Kogame. Tronoella, named in honor of Filipino phycologist Gavino C. Trono Jr., is a monotypic genus whose member is easily confused with the similarly amorphous and clathrate Hydroclathrus. The Okinawan endemic genus and species T. ryukyuana is distinguished from other similar Hydroclathrus species in having revolute branches that are occasionally inter-adhesive and are initiated from siphonous protrusions and in possessing conspicuous, highly protruding, and firmly coherent plurangia that are differentiated from cortical cells (Santiañez et al. 2018a). The species occupies the same intertidal habitat as that of Hydroclathrus and uses rhizoidal holdfasts to attach itself to the substrate (Santiañez 2018).
In their molecular-based taxonomic reassessment of some Scytosiphonaceae found in the Indo-Pacific Ocean, Santiañez et al. (2018b) described the new species Hydroclathrus rapanuii Santiañez, Macaya, & Kogame, based on Easter Island specimens that have long been identified as H. clathratus. Among the major takeaways in the studies of Santiañez et al. (2018a, 2018b) are the following: 1) there is a need to reassess the taxonomic identification of specimens previously identified as the supposedly cosmopolitan species H. clathratus, 2) the distribution of H. clathratus may not be as wide as earlier thought, and, 3) volcanic and isolated islands tend to host new species, and it is likely that assessment of their seaweed flora will result in the description of taxa new to science. Other studies of Hydroclathrus involved the taxonomy and nomenclature of H. stephanosorus. Based on morpho-anatomical analyses and integrating them with information derived from DNA phylogenies and Hydroclathrus distribution from Santiañez et al. (2018a, 2018b), Santiañez and Wynne (2019) proposed the synonymy of H. stephanosorus with the earlier described Talarodictyon tilesii [i.e., Hydroclathrus tilesii (Endlicher) Santiañez & M.J.Wynne], which Sinkora and Wynne (1990) synonymized with H. clathratus. More recently, Santiañez and Kogame (2022) segregated H. minutus from Hydroclathrus and established the new genus Manzaea [as Manzaea minuta (Santiañez & Kogame) Santiañez & Kogame] to account for the unique phylogenetic position and morpho-anatomy of ‘H. minutus’. Following this taxonomic change, Hydroclathrus is now reduced to five species (Table 1). Additionally, recent studies on the French Polynesia Scytosiphonaceae extended the distribution of M. minuta from the northwestern Pacific Ocean to the southeastern Pacific Ocean (Vieira et al. 2024)
Another interesting genus in the Scytosiphonaceae is Iyengaria, which is known to have a disjunct distribution in the Indian Ocean (Santiañez et al. 2020). The genus Iyengaria was established by Børgesen (1939) based on the branching and hollow brown seaweed collected from Dwarka, Gujarat, India, which he initially described as a new species of Rosenvingea [i.e., Rosenvingea stellata Børgesen (Børgesen 1928)]. Two years later, Børgesen (1930) transferred R. stellata to Colpomenia due to their morpho-anatomical similarities. Named in honor of the Indian phycologist Mandayam Osuri Parthasarathy Iyengar, the brown algal genus Iyengaria is distinguished from Colpomenia in having a semi-globular thallus with a semi-stellate appearance resulting from conical projections (Børgesen 1939). In establishing Iyengaria, Børgesen (1939) proposed the synonymy of the South African Colpomenia capensis Levring with the generitype Iyengaria stellata (Børgesen) Børgesen. However, it has been argued by Stegenga et al. (1997) that Co. capensis should be considered as a species distinct from I. stellata especially considering the peculiarly disjunct distribution of the species. Additionally, Wynne (1972) and Wynne and Norris (1976) pointed out the blurred genus-level morpho-anatomical delineation between Iyengaria and Colpomenia. As such, the status of the genus Iyengaria as a distinct genus has been repeatedly questioned. Nonetheless, two more species, Iyengaria nizamuddinii A.Abbas & M.Shameel and Iyengaria lobocylindrica Aisha & M.Shameel nom. inval., were later proposed.
The first molecular-based taxonomic treatment on an Iyengaria species was made by West et al. (2015) based on specimens from Tamil Nadu, India, which was described as a new species, Iyengaria quadriseriata J.A.West, Zuccarello, E.K.Ganesan & Loiseaux-de Goër. The results of the molecular phylogenetic analyses based on psaA data by West et al. (2015) showed that their I. quadriseriata represents a species that is closely related to Rosenvingea endiviifolia (Martius) M.J.Wynne [as Rosenvingea intricata (J.Agardh) Børgesen 1914] (Wynne and Nunes 2021). In the subsequent taxonomic, in vitro culture, and molecular phylogenetic studies on Iyengaria stellata from Kuwait and South Africa, Santiañez et al. (2020) made several taxonomic changes in the genus Iyengaria. Therein, Santiañez et al. (2020) confirmed the synonymy of Co. capensis with I. stellata, relegated I. nizamuddinii and I. lobocylindrica as junior heterotypic synonyms of I. stellata, and, based on phylogenetic and taxonomic reassessment synonymized I. quadriseriata with R. endiviifolia (as R. intricata). Following these taxonomic changes, Iyengaria is now considered as a monotypic genus (Table 1).
Jolyna S.M.Guimarães was originally established based on a single species of brown algae with an upright, foliose thallus with discoid holdfast (i.e., Jolyna laminarioides S.M.Guimarães) that was collected from Brazil (Guimarães et al. 1986). Named in honor of Brazilian phycologist Dr. Aylthon Brandão Joly, Jolyna has been considered as endemic to Brazil until Wynne and Banaimoon (1990) reported the occurrence of this species in the northern Arabian Sea. Similar to I. stellata, J. laminarioides also has a curious and disjunct distribution. Jolyna remained a monotypic genus for almost two decades until the description of the new species Jolyna furcata M.J.Wynne from Oman by Wynne (2003). Jolyna is considered to have very similar morpho-anatomies with Petalonia (including Endarachne) but is distinguished in having a medulla with numerous “fiber-like cells and the complex weft of interconnected filaments” (Guimarães et al. 1986). Despite several recent studies on the molecular phylogenetic relationships of the Scytosiphonaceae, Jolyna remained to be among the most understudied genus in the family. Considering the current state of the systematics of the Scytosiphonaceae, we advocate for a more detailed molecular-assisted taxonomic studies on Jolyna to confirm its segregation as a distinct genus as well as ascertain its phylogenetic position within the family. Nevertheless, we consider Jolyna as a distinct genus of two recognized species (Table 1).
The genera Melanosiphon and Myelophycus are morphologically similar in having caespitose, upright, and simple thalli that are initially solid and becoming hollow when older. Both genera are also distinct from other Scytosiphonaceae in exhibiting an isomorphic life history (Tanaka and Chihara 1984, Wynne 1969). The taxonomy and classification of species under Melanosiphon and Myelophycus have been reviewed by Tanaka and Chihara (1984), who suggested that 1) these are congenerics [i.e., Melanosiphon intestinalis (D.A.Saunders) M.J.Wynne should be transferred back to Myelophycus] based on widely overlapping morpho-anatomical characteristics, and 2) the genus Myelophycus should be classified under Dictyosiphonaceae (Dictyosiphonales). After assessing the reproductive biology of Myelophycus simplex (Harvey) Papenfuss, Kawai et al. (1994) suggested that Myelophycus should be classified under Scytosiphonaceae (Scytosiphonales). This classification was confirmed by the molecular phylogenetic work of Cho et al. (2003), who also emended the description of the family Scytosiphonaceae to include the isomorphic life history of the genus Myelophycus. Concerning Melanosiphon, Wynne (1990) argued that the genus is distinct from Myelophycus in 1) having thinner cortex and medulla that renders them flaccid when taken out of the water as well as in 2) possessing paraphyses (= assimilatory filaments) with longitudinal divisions. The first molecular phylogenetic basis on the segregation of both genera was provided by Lee et al. (2014a) and confirmed by McDevit and Saunders (2017) through their multigene phylogenetic tree.
Petalonia species are distinguished from other morphologically similar Scytosiphonaceae by their flattened, solid, and foliose thalli possessing medullary rhizoidal filaments that are entangled (Kogame et al. 1999). As with other scytosiphonacean genera, the phylogenetic relationships of Petalonia species were not monophyletic following the work of Kogame et al. (1999). It was not until the studies on the Scytosiphon-Petalonia complex of Canada by McDevit and Saunders (2017) that some taxonomic changes were facilitated in the genus. Therein, McDevit and Saunders (2017) showed that Scytosiphon complanatus (Rosenvinge) Doty, Scytosiphon gracilis Kogame, and Petalonia zosterifolia (Reinke) Kuntze, which exhibit thalli that are compressed to flattened and are hollow to partially hollow, formed a highly supported monophyletic clade separated from other members of Petalonia and Scytosiphon. By integrating morpho-anatomic data with molecular phylogenetic information based on the principle of monophyly, McDevit and Saunders (2017) segregated S. complanatus, S. gracilis, and P. zosterifolia, and established the new genus Planosiphon McDevit & Saunders, with P. complanatus as the generitype. Planosiphon species are identified based on their upright, compressed to flattened, and hollow to partially hollow thalli that lack ascocysts. Following the establishment of Planosiphon, Santiañez and Kogame (2017) transferred Petalonia filiformis to the genus [as Planosiphon filiformis (Batters) Santiañez & Kogame] and emended the description of the Planosiphon to include the reported life history of its known members, that is, having prostrate, Compsonema-like sporophytic thalli bearing only unangia. Currently, the genus Planosiphon includes four species (Table 1). Following the lead of McDevit and Saunders (2017), Santiañez and Kogame (2019) reassessed the taxonomy and molecular phylogenetic position of Scytosiphon tenellus Kogame, a species collected from Muroran, Hokkaido, Japan, and described based on taxonomic and life history studies (Kogame 1998). Based on their molecular phylogenetic studies on S. tenellus specimens from Muroran, the species formed a highly supported monophyletic clade with other Petalonia species; as such, Santiañez and Kogame (2019) transferred S. tenellus to the genus Petalonia [now known as Petalonia tenella (Kogame) Santiañez & Kogame]. Santiañez and Kogame (2019) also noted that all Petalonia species (including P. tenella that they recently transferred) exhibit a Stragularia-like sporophytic thalli that bear only unangia. They considered these characteristics as taxonomically important [similar to those earlier suggested by Kogame et al. (1999) and Cho et al. (2006), among others] and expanded the description of Petalonia to include the morpho-anatomy of their prostrate sporophytic thalli. Currently, there are five recognized species of Petalonia, most of which are found in the Pacific Ocean (Table 1).
Species classified under Rosenvingea have hollow thalli that are erect, freely to inter-adhesively branching, cylindrical to somewhat compressed (Børgesen 1914, Norris 2010, West et al., 2010, Lee et al. 2014a, Taylor 1960). The taxonomy of Rosenvingea species is known to be problematic, especially those of the erect species as they are primarily distinguished through their branching patterns (Norris 2010). As such, Norris (2010) has advocated the use of molecular data to define the erect species of Rosenvingea. West et al. (2010) were the first to study the life history along with the molecular phylogenetic placement of Rosenvingea orientalis (J.Agardh) Børgesen within the Scytosiphonaceae. Therein, they noted that two distinct lineages identified as R. intricata (now currently known as R. endiviifolia), collected from Japan and New Caledonia, are not monophyletic. In the subsequent study of Lee et al. (2014b), they recovered four Rosenvingea lineages in the psaA gene-based phylogenetic tree. Three of these lineages were identified as R. endiviifolia (as R. intricata), which were recovered as a well-supported clade. However, no detailed morpho-anatomical characterizations were provided in their work. In their reassessment of the Rosenvingea from Western Australia, Huisman et al. (2018) recovered two distinct lineages that clustered with R. orientalis from Vietnam and Mexico, and ‘R. intricata’ from New Caledonia. Based on phylogenetic and morpho-anatomical observations, they proposed the latter lineage as the new species Rosenvingea australis Huisman, G.H.Boo & S.M.Boo consequently resolving the problematic identity of those presumed to be R. endiviifolia (as R. intricata). Studies on the life history in culture and phylogeny of Rosenvingea from the Philippines by Santiañez and West (2019) extended the distribution of R. australis to the Philippines. Their herbarium collections-based observations on unknown Rosenvingea from Puerto Galera, Philippines, suggested the presence and range extension of R. nhatrangensis E.Y. Dawson to the country (Santiañez and West 2019). Vieira et al. (2024), in their latest molecular-assisted taxonomic studies on the Scytosiphonaceae in French Polynesia, have shown polyphyly within the genus Rosenvingea especially considering the phylogenetic positions of the two new species that they recently described (i.e., Rosenvingea polynesiensis C.W.Vieira & M.Zubia and Rosenvingea tahitiensis C.W.Vieira & M.Zubia). Nonetheless, considering the extensive mis-identifications in Rosenvingea, especially the supposedly widely distributed R. endiviifolia, points to the need to conduct studies on the types and thorough taxonomic reassessment of the genus.
The members of the genus Scytosiphon typically possess a cylindrical to compressed hollow thallus with plurangia interspersed with ascocysts. Petalonia species share the similar habit of an upright thallus with Scytosiphon species. Considering the wide morpho-anatomical variations within these genera, it has long been accepted that species classified under Scytosiphon and Petalonia have intermediate characteristics (Kogame et al. 1999, McDevit and Saunders 2017).
McDevit and Saunders (2017) showed that Scytosiphon is not monophyletic, suggesting that the traditional genus-level definitions based on morphological characters were unreliable. As mentioned earlier, to resolve some of the problems in the taxonomy and molecular phylogeny within the Scytosiphon-Petalonia complex, and to adhere to the principle of monophyly, McDevit and Saunders (2017) proposed the new genus Planosiphon. In the same work, McDevit and Saunders (2017) also noted the problem surrounding the identity of Scytosiphon canaliculatus (Setchell & N.L.Gardner) Kogame. Molecular phylogenetic studies including S. canaliculatus showed its segregation from its congeners. Previously classified as the generitype of Hapterophycus, S. canaliculatus was transferred from Hapterophycus to Scytosiphon by Kogame (1996) based on his studies on the morpho-anatomy and life history of the species from Hokkaido, Japan. Santiañez and Kogame (2019), however, pointed out that the reproductive biology and morphology of the sporophyte of S. canaliculatus is different from other Scytosiphon species. Integrating this information with molecular phylogenetic data, Santiañez and Kogame (2019) returned S. canaliculatus to its original genus assignment (as Hapterophycus canaliculatus Setchell & N.L.Gardner). The genus Scytosiphon remains to be among the most diverse within the Scytosiphonaceae and now includes eight known species (Table 1).
In the most recent systematic treatments of the Scytosiphonaceae, Santiañez (2018, 2023) proposed further changes in the classification in the family based on information derived from their molecular phylogeny, ecology and distribution, and life history and reproduction. The phylogenetic segregation of the family Scytosiphonaceae has been noted since the work of Kogame et al. (1999), and this has been repeatedly seen in subsequent single- and multigene-based molecular phylogenetic studies (e.g., Cho et al. 2006, Huisman et al. 2018, Lee et al. 2014a, McDevit and Saunders 2017, Santiañez et al. 2018a, 2018b, 2020, Santiañez and Kogame 2019, 2022, Santiañez and West 2019). Kogame et al. (1999) were the first to outline some of the similarities and differences among members of the two minor clades (Group 1 and Group 2) and two major clades (Group 3 and Group 4) in the Scytosiphonaceae. Group 3 included Petalonia, Scytosiphon [which also represented taxa from Hapterophycus and the newly segregated genus Planosiphon, and elongate Colpomenia (now known as Dactylosiphon)] and Group 4 is consisted of Pseudochnoospora [as represented by Pseudochnoospora implexa (as C. implexa), Rosenvingea, Hydroclathrus and the sac-like Colpomenia]. Kogame et al. (1999) suggested that differences in temperature preference (hence, distribution patterns), morphology of prostrate sporophytic thalli, their reproductive organs, along with molecular phylogenies can be used as criteria in facilitating revisions within the group. Cho et al. (2006) floated the idea of segregating the family Scytosiphonaceae into two tribes, ‘Scytosiphonieae’ and ‘Chnoosporieae’ or lumping them into two large genera, Scytosiphon and Hydroclathrus. However, Cho et al. (2006) did not formalize any of these proposals as the former is constrained by the extensive paraphyly in the family, whereas the latter cannot account for the isomorphic life histories of Myelophycus and Melanosiphon.
Considering all the changes he introduced in his studies on the taxonomy and molecular phylogeny of the Scytosiphonaceae [as formalized in several publications such as Santiañez et al. (2018a, 2018b), Santiañez and Kogame (2019, 2022) and Santiañez (2022)], Santiañez (2018) proposed the recognition of two new tribes within the Scytosiphonaceae: Hydroclathreae Santiañez and Scytosiphoneae Santiañez. However, these proposals are considered invalid as these were not published according to the requirements of the International Code of Nomenclature (ICN) for algae, fungi, and plants (Turland et al. 2018, Santiañez 2023). Additionally, the name Scytosiphoneae, as Santiañez (2023) would later realize, had already been used to refer to a phyletic group at the tribe level and that the name should be attributed to Thuret (in Le Jolis 1863: 14). Nonetheless, Santiañez (2023) formally emended the Tribe Scytosiphoneae Thuret to include scytosiphonacean taxa that are found primarily in cold waters and possessing an alternation of isomorphic and heteromorphic generations, and whose microscopic sporophytes produce only unangia. In the same work, he also formally established the Tribe Hydroclathreae Santiañez, to include scytosiphonacean taxa with affinities to warmer waters, exhibiting an alternation of heteromorphic generations, and having both unangia and plurangia in their microscopic sporophytic thalli. The most recent classification of the members of the Scytosiphonaceae is outlined in Table 1.
Systematic Revisions in the Scytosiphonaceae: The way Forward
Recent taxonomic revisions in the taxonomy, classification, and nomenclature in the Scytosiphonaceae as facilitated by molecular phylogenetic studies have greatly contributed to resolving the decades-old and complex challenges within the family. Indeed, the problems associated with the systematics of the Scytosiphonaceae have provided an interesting case on the utility of molecular tools in addressing issues related to algal taxonomy and classification. Recent taxonomic and molecular phylogenetic studies in the Scytosiphonaceae also underscore the need to define (brown) seaweed genera and species beyond their gross morphologies; rather, it is necessary to take into account several features including internal anatomies, reproductive structures, morpho-anatomies of microscopic stages (if any), and molecular phylogenetic affinities (ideally based on multigene phylogenies). As with other algal groups, several new genera (6) and species (11) of Scytosiphonaceae have been described primarily from the subtropical to temperate waters (i.e., tribe Scytosiphoneae). Considering this, we advocate shifting our focus on tropical members (i.e., tribe Hydroclathreae), especially the genera Rosenvingea and Colpomenia. We expect that more detailed integrative studies on these and other closely related genera will result in descriptions of new taxa at various phyletic levels.
We also note the apparent proliferation of new genera within the tribe Hydroclathreae, which are consequences of attempting to conduct ‘taxonomic housekeeping’ within a group that used to be extremely polyphyletic and paraphyletic. While these bold taxonomic changes—which admittedly follow a pragmatic framework informed by molecular data—appear to resolve some of the glaring problems in the systematics of the Scytosiphonaceae, we also advocate caution on the possible taxonomic inflation in the family. Impacts of taxonomic inflation among seaweed groups may vary in scale, but it may have negative implications to marine macroecology and conservation of marine resources and environment, especially that conservation and protection plans are informed by species attributes includeing richness, diversity, and endemism, among others (Isaac et al. 2004). Nevertheless, we believe that a thorough review of the taxonomies and molecular phylogenetic relationships of scytosiphonacean taxa will be beneficial towards building better knowledge, understanding, and ultimately provide a natural classification in the Scytosiphonaceae.