Introduction
Methods
Sites for investigation
Data collection and treatment
Results
Discussion
Taxonomic Revisions and Implications
Need for Expanded Field Surveys
Biogeographic Patterns and Diversity Drivers
Applications of Molecular Biology
Introduction
Nestled along the central Vietnamese coast, the Hai Van - Son Cha is a remarkable Marine Protected Area (MPA) designated for its exceptional biodiversity and ecological significance. Hai Van - Son Cha, strategically positioned between distinct northern and southern biogeographic zones, exhibits a fascinating overlap of species originating from differing climatic regions. Its biogeographic position allows unique opportunities to investigate ecological and evolutionary processes shaping species distributions, adaptations, and interactions along this biogeographic boundary. This area encompasses a mosaic of diverse ecosystems, including lagoons, sandy shores, rocky shores, seagrass beds, coral reefs, and islands. The diversity of intertidal and subtidal habitats has nurtured rich marine life with 1084 marine species belonging to 537 genera, 238 families, 31 classes, designating Hai Van - Son Cha as one of Vietnam's first 16 marine protected areas in 2014 (Nguyen et al. 2023, Yet and Tien 2004). This mixing of distinct biotas creates a fascinating blend of species that support abundant. These unique environmental and ecological characteristics of Hai Van - Son Cha offers exceptional research value in terms of marine science.
A key component of this rich biodiversity is the marine macroalgae. These conspicuous, multicellular algae play a pivotal role as ecosystem engineers, shaping the ecological processes that underpin the health and function of coastal waters (e.g., Corrigan et al. 2022, Cotas et al. 2023). Their diverse assemblage directly influences primary production, the foundation of marine food webs, and facilitates crucial nutrient cycling processes, ensuring the availability of essential elements for various organisms. Additionally, their abilities of stabilizie sediments and carbon storage are preventing coastal erosion and mitigating the detrimental effects of land-based activities. Furthermore, the architectural complexity of macroalgal assemblage provide a critical structured habitat for a plethora of associated species, offering refuge, breeding grounds, and a source of nourishment (Cotas et al. 2023). Consequently, healthy and diverse macroalgal communities contribute significantly to the overall ecosystem services and maintaining the resilience of the coastal environment.
The significance of marine macroalgae is undeniable, research efforts in Vietnam have primarily focused on taxonomic identification basis of morphology mainly and biogeographical distribution since the 1930s (Ho 1969), syntheses of contemporary data are lacking (e.g., molecular, SEM,..). While the Hai Van - Son Cha region has been a strong point in the field of phycological research for decades, the last comprehensive survey was over 20 years ago (from 1970s-2004) (Yet and Tien 2004, Dam 2006). Since then, there have been no continual advances in algal taxonomy and systematics conducted by new field studies in Hai Van - Son Cha. Therefore, updated baseline data on macroalgal species composition is critically needed.
Hai Van - Son Cha, there becomes even more important as the area is a biodiversity hotspot, provides an invaluable natural laboratory to examine complex ecological relationships and dynamics between organisms and their environments across multiple ecosystems (Do 2022, Long and Tuan 2014, Trung et al. 2023). Documenting biodiversity in MPAs provides important data for ecological, ecosystem and evolutionary research, helps assess environmental impacts, develop management and conservation strategies, and raise awareness of biodiversity, education and support for sustainable development. This is not only important for scientific purposes but also for long term environmental and social benefits.
Therefore, the aims of this study are: (1) to systematically review existing literature and compile an updated checklist of marine macroalgae species reported from field studies along the Hai Van - Son Cha coastline and (2) to highlight changes in taxonomic nomenclature since past surveys and discuss biogeographic diversity patterns along this heterogeneous coastline. Achieving these goals will establish a benchmark for assessing long-term biotic changes through continued ecological monitoring and expanded biodiversity surveys in this vital conservation area.
Methods
Sites for investigation
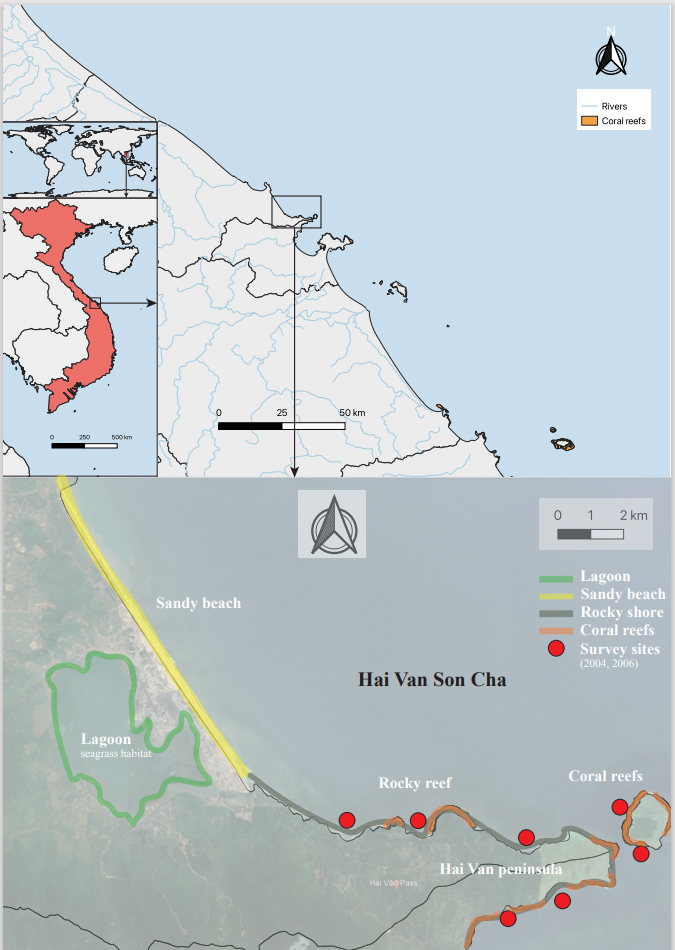
The Hai Van - Son Cha Marine Protected Area (MPA) is situated in central Vietnam, spanning from 108°04'09" to 108°13'56" longitude and 16°11'29" to 16°15'34" latitude. Encompassing an area of 10.265 hectares, the MPA boasts a remarkably diverse geography. Its northern border adjoins Thua Thien Hue province at Lap An Lagoon, while the southern boundary meets Da Nang City. The coastline is predominantly rugged, with approximately 14 km of steep cliffs and rocky reefs. This unique location at the confluence of northern and southern biogeographic zones subjects the MPA to a temperate, humid climate with mild winters and hot summers. The temperature, humidity, rainfall, and semi-diurnal tidal regime all contribute to the region's exceptional ecological diversity. The MPA encompasses a mosaic of ecosystems, including coral reefs, seagrass beds, intertidal zones, mangrove forests, rocky shores, sandy beaches, and islands, providing habitat for a wide array of marine life (Fig. 1).
Data collection and treatment
To investigate the macroalgal diversity along the Hai Van - Son Cha coastline, we searched Google Scholar (https://scholar.google. com), Web of Science (https://clarivate.com/webofsciencegroup), ScienceDirect (https://www.sciencedirect.com), Scopus (https:// www.scopus.com) and other online databases for published studies that reported species lists and occurrence data from field surveys in this area. We also reviewed historical taxonomic studies. The last step was to obtain unpublished works such as these for M. Phil. and Ph.D. and Technical Reports from universities and scientific institutions in Vietnam S&T publication database (https://sti.vista.gov.vn). We selected the studies that met the following criteria: (1) they were published in peer-reviewed journals, conference proceedings, project reports, or summary reports, (2) reported species lists from field collections made along the Hai Van - Son Cha coastline, and (3) they focused on the Hai Van - Son Cha coastline. From the selected studies meeting these criteria, the following data were extracted into a database: species names, higher taxonomic classifications, sampling locations along the coastline, years of collections, and methodological details including habitats sampled. In total, five studies published between 1992 and 2012 were reviewed.
Species names were updated to currently accepted names and classifications using Guiry and Guiry (2024) and other taxonomic databases. Synonyms and taxonomic changes were noted. The updated checklist was summarized by higher taxa. Unique and shared species between studies were assessed to elucidate sampling completeness. Biogeographic patterns were analyzed by comparing species distributions along the coastline.
The current taxonomic status and nomenclatural changes of the taxa of interest have been considered following Algaebase (https://www.algaebase.org/) (Guiry and Guiry 2024). Phyla, class, orders, families, genera and species are alphabetically arranged. To classify the Hai Van – Son Cha algae flora, we have applied Feldmann’s ratio [R/O] (number of species of Rhodophyta/number of species of Ochrophyta) and Cheney’s ratio [(R+C)/O] in a similar manner to Feldmann’s but including green algae (Cheney 1977, Feldmann and et Lami 1937) respectively. These ratios indicate the position of macroalgae flora in relation to tropical or subtropical.
Results
This systematic review synthesizes findings from five pertinent studies published between 1992 and 2006 (Yet and Tien 2004, Dam 2006), which report on the macroalgal diversity observed during field surveys conducted specifically along the Hai Van - Son Cha coastline. These studies encompassed 16 distinct sites along a stretch of approximately 25 km of shoreline between Northern Hai Van and Son Cha. The methodologies employed varied across the studies, including quadrat sampling, line transects, and collections performed while snorkeling or SCUBA diving, to survey intertidal and shallow subtidal habitats. Complementing this, the assessment incorporates data from additional studies conducted from 2012 to 2023 (Hoai et al. 2012, Cuong et al. 2015, Tran 2023), which documented research samples of macroalgal species collected within the same marine area. This dual approach ensures a robust and comprehensive analysis of the region’s macroalgal diversity over an extended period.
Across the five reviewed studies, an initial compilation yielded the list of marine species belonging to four phyla including Rhodophyta, Ochrophyta, Chlorophyta and Cyanobacteria reported from Hai Van - Son Cha area. Based on the previous studies, we have collected a total of 142 species from 23 orders, 38 families, 54 genera of 4 phyla. Among them, Rhodophyta has recorded the highest species number (72 species), followed by Ochrophyta (39 species), Chlorophyta (23 species) and Cyanobacteria (8 species). These data indicate the high biodiversity of the study area, especially for red algae.
After updating the systematic classification according to the current scientific names, the list has been revised with 134 species from 22 orders, 36 families, 63 orders of 4 phyla. Compared to the initial list, 9 species were removed due to duplication or invalidity, and 10 orders were added due to being split from other order. Rhodophyta has still the highest species number (67 species), but there are changes in the number of families (decreased from 20 to 19) and order (increased from 28 to 38). The species number of Ochrophyta has changed (38 species instead 39), has not changed their number of orders (12 orders). In the Chlorophyta, there has been changed in order number (decreased from 11 to 10), but the number of species remained the same with (21 species). Cyanobacteria has not changed in the number of species (8), families (3) and order (3). This indicates that Vietnam has a high floristic affinity with the tropical regions, as expected from its geographical location (Garbary 2001).
Among updated species, a total of 50 species from the original list were updated (37%). Rhodophyta (red algae) represent the most taxon-rich group with 28 species from 10 order, 14 families and 22 genera. Ochrophyta (brown algae) contribute 11 species from 4 order, 4 families and 6 genera. Chlorophyta (green algae) account for 7 species from 3 order, 4 families and 5 genera, Cyanobacteria for 1 species from 1 order, 1 family and 1 genus. Other key results from the updated checklist include: One species of Chlorophyta was removed from the checklist due to the misspelled name (i.e., orthographic mistake in AlgaeBase) “Codium tunue”, which is an invalidly published name; one species within the Ochrophyta was excluded due to a lack of taxonomic clarity regarding the Laminaria sp., typically found in temperate and sub-polar regions; two potentially misidentified species needing confirmation via new collections (Sargassum spp.); species assigned to three genera (i.e. Asterocytis, Gelidiopsis and Enteromorpha) have currently transferred to other genera (i.e. Chroodactylon, Ceratodictyon, and Ulva, respectively); four species names duplicated in the old list of Yet and Tien (2004), Dam (2006) such as “Acrocystis nana, Ceramium clarionens, Hypnea cervicornis, and Padina boryana”; and four species names were incorrectly identified due to typing errors in the cases of “Acrocystis ornata, Lithophyllum okamurai, Lithophyllum thrichotomum, Grateloupia divaricatum”.
A full checklist showing previously reported names and updated accepted names is provided in Table 1.
Table 1.
Checklist of macroalgae of the Hai Van - Son Cha
Note: a - Yet and Tien (2004), b - Dam (2006), c - Hoai et al. (2012), d - Cuong et al. (2015), ND – No data
The data presented in Table 2 suggests a temporal decline in both Cheney’s and Feldmann’s ratios within the Hai Van - Son Cha region, with Cheney’s ratio decreasing from 2.63 in 2004 to 2.32 in 2024, and Feldmann’s ratio dropping from 1.94 to 1.76 over the same period. This trend could be indicative of an increase in Chlorophyta diversity or a decrease in Rhodophyta diversity. Alternatively, it may reflect changes in the methodologies or criteria employed in these studies over time. Notably, the marine flora of Hai Van - Son Cha is characterized by a predominance of red algae (Rhodophyta), which underscores the richness and diversity of this group in tropical marine environments. The observed changes in Cheney’s and Feldmann’s ratios warrant further investigation to elucidate the underlying ecological dynamics and methodological influences in this biodiverse region.
Table 2.
Number of species and percentages of each algae group in the Hai Van - Son Cha, including Cheney´s ratio and Feldmann’s ratio
| Study | Rh | Och | Chl | Cya | Total | Cheney’s ratio | Feldmann’s ratio |
| Yet and Tien (2004) | 68 | 35 | 24 | 8 | 135 | 2.63 | 1.94 |
| Dam (2006) | 50 | 26 | 16 | 5 | 97 | 2.54 | 1.92 |
| This study | 67 | 38 | 21 | 8 | 134 | 2.32 | 1.76 |
|
Overall Vietnam updates up to 2023 (Dam et al. 2023, Nguyen et al. 2023) | 439 | 166 | 196 | 87 | 888 | 3.83 | 2.64 |
Discussion
This systematic review has yielded a comprehensive and taxonomically updated checklist of 134 marine macroalgal species from the Hai Van-Son Cha coastline in central Vietnam. The updated checklist, containing 134 species from 22 orders, 36 families, and 63 genera of 4 phyla, provides an important baseline for future monitoring and research. Representing a significant contribution to the existing knowledge of Vietnam's marine biodiversity, this number constitutes approximately 15% of the total macroalgal species reported in Vietnam to date (Dam et al. 2023, Nguyen et al. 2023). The importance of this finding is further amplified when considering the surface area involved. The Hai Van-Son Cha coastline encompasses only 10.265 ha, which represents a mere 0.011% of Vietnam's total surface area (approximately 100,000,000 ha). That such a small region harbors such a disproportionately high diversity of macroalgae (over 15% of the national total) highlights its status as a critical biodiversity hotspot and emphasizes the need for its conservation and sustainable management.
Comparisons between the studies revealed several species found across all surveys, indicating widespread and common species along this coastline. However, there was variation in the specific species reported from each site and habitat type. Approximately 10.4% (14 species) of species were unique to single studies, highlighting the value of expanded sampling effort to approach a more complete inventory. The compiled updated checklist provides an important baseline understanding of marine macroalgal diversity across Hai Van-Son Cha. The taxonomic revisions demonstrate the need for periodic reviews to track nomenclature changes. Additional collections and surveys are still needed to build upon this synthesis.
This study reveals the presence of invasive species such as Asparagopsis taxiformis (Delile) Trevisan 1845 originating from Australia (Andreakis et al. 2016) and Acanthophora spicifera (M.Vahl) Børgesen originating from the Indian Ocean and detected the first in Guam (Weijerman et al. 2008).
When compared with the studies in the neighboring regions, the marine flora of Hai Van - Son Cha is similar to the marine flora of Con Co (Dam et al. 2021), Co To - Thanh Lan (Dam et al. 2020), Phu Yen (Hang et al. 2021) and Nam Du (Do et al. 2019). This could suggest that these regions share similar geographical, environmental and historical factors that shape the marine flora.
Intriguingly, our investigation sheds light on potential underestimations of specific taxa. While various studies have documented the remarkable diversity of marine macroalgae in Vietnam, the true extent of this richness may be significantly underappreciated. This is exemplified by the genus Lobophora, which has been the subject of recent studies in areas neighboring our research site. These studies identified a staggering nine new records and two new species of Lobophora in Con Co island, a stark contrast to the single species previously reported in Vietnam. This significant discrepancy suggests widespread underestimation of Lobophora's distribution and diversity across the country. Our findings mirror this underestimation, as we documented only one Lobophora species. Similarly, recent research focusing on other taxa, like Ulva (Tran et al. 2022a, Tran et al. 2022b), Dictyota (Nguyen et al. 2019), Phycocalidia (Zhao et al. 2021) and Graterloupia and Phyllymenia (Nguyen et al. 2024), has revealed numerous new records and species, further highlighting the potential for underrepresentation across various macroalgal groups by molecular re-assessment (an advanced molecular biology tool, necessary in current taxonomy in the world).
These observations point towards the need for a paradigm shift in our understanding of Vietnam's marine macroalgal diversity. Our study, along with recent findings from neighboring regions, suggests that the current knowledge base likely underestimates the true richness and complexity of these communities.
This necessitates continued research efforts, including: (i) Continue to update modifications and classification meanings; (ii) Comprehensive surveys; (iii) Refined taxonomic scrutiny; (iv) Targeted studies by biogeographic patterns and diversity drivers.
By addressing these knowledge gaps, we can gain a more comprehensive understanding of the remarkable biodiversity harbored within Vietnam's coastal ecosystems and their vital roles in the global ecological landscape. This knowledge will pave the way for the development of effective conservation strategies and sustainable management practices, ensuring the long-term health and resilience of these critical marine environments.
Taxonomic Revisions and Implications
Algal taxonomy and nomenclature are dynamic fields that are constantly evolving due to the advances in molecular and morphological methods, as well as the discovery of new taxa and the revision of existing ones. The International Code of Nomenclature for algae, fungi, and plants is the set of rules and recommendations that govern the scientific naming of algae, fungi, and plants, and it is periodically updated to reflect the changes in taxonomic knowledge and practice (Turland, et al. 2018).
The taxonomic revisions made through this review are based on the latest literature and databases, such as AlgaeBase (Guiry and Guiry 2024), WoRMS (Ahyong et al. 2024), and they involve changes in taxon concepts, reclassifications of taxa, and paradigm shifts reflected in naming conventions. Many macroalgal taxa have complex taxonomic and nomenclatural histories, for example, the genera names Pachymeniopsis Yamada and Prionitis J. Agardh had been merged into Grateloupia C. Agardh (Kawaguchi 1997, Wang et al. 2001), and then reinstated via recent taxonomic reassessment (Gargiulo et al. 2013), Dilophus J.Agardh, 1882 synonymized with Dictyota J.V.Lamouroux, 1809 (Bogaert et al. 2020), Enteromorpha Link, 1820 with Ulva Linnaeus, 1753 (Hayden et al. 2003, Mantri et al. 2020), Asterocytis (Hansgirg) Gobi ex F.Schmitz with Chroodactylon Hansgirg (Guiry and Guiry 2024), and Gelidiopsis F.Schmitz with Ceratodictyon Zanardini (Schneider and Wynne 2007).
The taxonomic revisions and implications for the marine algae of Hai Van - Son Cha are important for biodiversity cataloguing, as they provide an updated and accurate checklist of the algal species and their current names and classifications. This checklist can serve as a useful reference for future studies and conservation actions on the marine flora of Hai Van - Son Cha and other regions of Vietnam. The checklist can also help to identify native, alien, endemic, rare, threatened, and endangered marine algal species in Hai Van - Son Cha. The name changes documented here are critical for comparing past and present surveys and avoiding misleading conclusions about species losses or gains due to taxonomy alone. By using the current names and classifications of the algal species, the comparison of the surveys can be more reliable and consistent, and the changes in the species diversity and distribution can be more accurately attributed to the environmental and anthropogenic factors, rather than the taxonomic ones. For example, by using the current name of Sphacelaria rigidula Kützin, instead of the old name of Sphacelaria furcigera Kützing or Sphacelaria variabilis Sauvageau, the comparison of the surveys can avoid the false impression that Sphacelaria furcigera and Sphacelaria furcigera have disappeared from Hai Van - Son Cha, when in fact it is still present under a different name.
Need for Expanded Field Surveys
The need for expanded field surveys is evident due to the varying species reported in different studies, indicating that there is a lack of comprehensive understanding of the macroalgae diversity along the Hai Van - Son Cha coastline. On average, 10.4% of the species were unique to each study, suggesting that the total diversity has not been fully captured. To achieve a more complete inventory, it is necessary to conduct additional collections in unsurveyed sites, habitats, and seasons. Furthermore, there is a need to verify the identification of two unidentified species (Sargassum spp.) and re-examine the distribution of Lobophora vieigarta in the Indo-Pacific Ocean (Dam et al. 2023, Vieira et al. 2016). The purported presence of Laminaria sp. along the Hai Van - Son Cha stretch, as mentioned in Tran's (2023) Ph.D thesis, lacks empirical evidence and requires verification. A more detailed study of Grateloupia and its relationship with Phyllymenia is also recommended (De Clerck et al. 2005, Kim et al. 2021). The species accumulation trends from the collective studies suggest that diversity is approaching saturation, but further sampling is likely to reveal additional rare or patchily distributed species. It is also worth noting that certain taxa, such as Rhodophyta and Cyanobacteria, may be underrepresented and require more extensive collection efforts.
Biogeographic Patterns and Diversity Drivers
The biogeographic patterns offer valuable insights into the underlying factors that drive biodiversity, particularly in marine macroalgae. The intricate ecosystems along coastlines serve as a microcosm for observing these patterns and understanding their implications. In the Hai Van – Son Cha area, the presence of complex rocky reef habitats has been associated with higher species richness and the formation of unique species assemblages. This contrasts sharply with the more uniform soft-bottom sandy habitats, which tend to support fewer species. Such observations underscore the importance of habitat heterogeneity as a critical driver of algal diversity. This principle is not unique to this region; it resonates with findings from other coastal systems across the globe, as also evidenced in other coastal systems worldwide (Mulders et al. 2022, Pearman et al. 2023). Understanding these diversity-environment relationships can help target additional sampling and guide management. Recognizing the role of habitat complexity in fostering biodiversity is crucial for conservation efforts. It can inform targeted sampling strategies, ensuring that areas of high diversity and ecological significance are identified and studied in greater detail. Moreover, this understanding can guide effective management practices, aiming to preserve the intricate balance of these ecosystems and the diversity they support (Anderson et al. 2009). By delving into the relationship between environmental variables and biodiversity, researchers can predict potential changes in ecosystems resulting from natural or anthropogenic disturbances (Jung and Choi 2022). This predictive power is essential for developing proactive conservation strategies that can mitigate the impacts of such disturbances and maintain the resilience of marine macroalgal communities.
Applications of Molecular Biology
Emerging molecular biology tools can significantly advance future cataloguing of Hai Van - Son Cha algal diversity. DNA barcoding using standardized gene markers allows species delimitation and identification where traditional taxonomy is difficult (Bartolo et al. 2020, Gary et al. 2010, McDevit and Saunders 2009). According to the latest Guiry 2024 publication in which molecular methods have led to an expansion of the number of genera in Cyanobacteria, including 47 new species described on average each year over the past 10 years. In contrast, brown algae (Ochrophyta) and red algae have 16 and 77 species, respectively, described (Guiry 2024). Comparative phylogenetics can elucidate evolutionary relationships and biogeographic affinities (Andreakis et al. 2016, Bringloe et al. 2020, Vieira et al. 2021, Zhao et al. 2021). The application of these molecular approaches will overcome the inherent limitations of morphology-based classification and accelerate the discovery of the full diversity of marine algae species along the Hai Van - Son Cha coast. Drawing a parallel with higher flowering plants, the APG IV system represents the fourth version of the modern, largely molecular-based plant classification system. It builds on the APG III system by only introducing changes when there is a “well-supported need.” This led to the recognition of five new orders: Boraginales, Dilleniales, Icacinales, Metteniusales, and Vahliales and several new families, culminating in a total of 64 angiosperm orders and 416 families (Chase et al. 2016). In light of these advances, future research on modern algal taxonomy must also incorporate both morphological anatomical and molecular data. This dual approach will ensure a more comprehensive and accurate classification, reflecting the true diversity and complexity of algal species.