Introduction
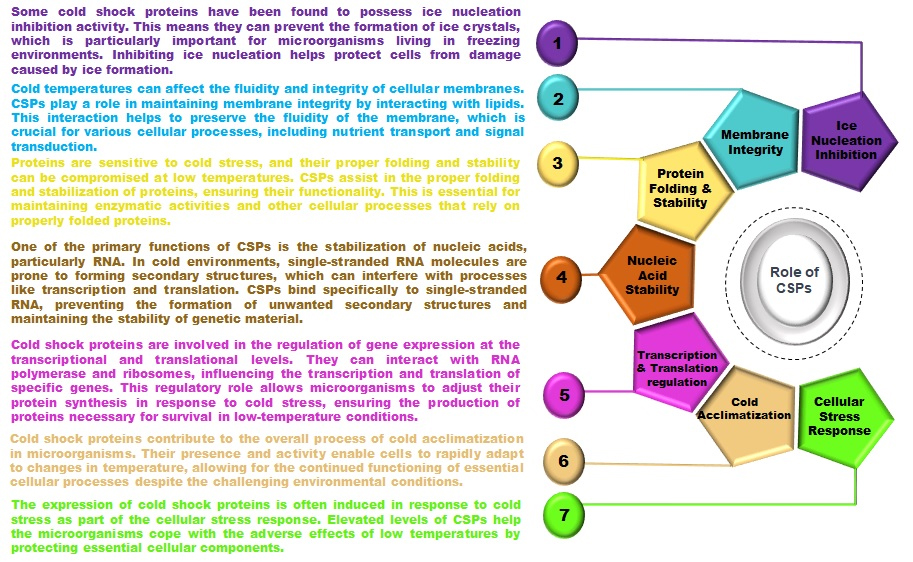
Biophysical of Cold-Shock Proteins
Structural Feature of Cold Shock Proteins
Cold Shock Domain (CSD)
β-strands arrangement
RNA-Binding Site
Flexibility and functional versatility
Oligomeric structures formation
Conclusion
Introduction
Temperature plays a pivotal role in determining the survival of living organisms, presenting heightened challenges in frigid environments such as the cryosphere. In this frozen realm, encompassing ice sheets, glaciers, and frozen surfaces, microorganisms like bacteria, algae, protozoa, and viruses confront substantial hurdles to their existence. Solid ice forms an inhospitable environment for microbes, rendering them incapable of surviving within its frozen structure.
Living in a cold environment poses fundamental challenges as low temperatures impede crucial biological processes, including nutrient movement and chemical reactions. Psychrophiles address these challenges by employing adaptive strategies. They enhance the number of transporters on their cell surfaces, actively capturing nutrients from surrounding water to ensure a sufficient food supply. Another significant challenge in frozen environments is the formation of ice crystals. Analogous to the human body, which is primarily composed of water, bacterial cells also contain a significant water content that can crystallize at freezing temperatures. Ice crystals within living cells pose a threat, growing outward like thorns and ultimately piercing the cells, leading to their demise. Psychrophiles have evolved various strategies to counteract the dangers associated with ice formation. They intake dissolved salts and sugars, lowering the freezing temperature of the water inside their cells.
Some psychrophile species produce special antifreeze proteins and cold shock response strategies that attach to forming ice crystals, lowering their freezing point and impeding their growth (Jones and Inouye 1994). Through these adaptive mechanisms, psychrophiles mitigate the risks associated with living in cold environments, showcasing the remarkable resilience and versatility of life in extreme conditions (Fig. 1). Both prokaryotic and eukaryotic cells exhibit an adaptive cold shock response when exposed to a sudden temperature decrease (Bartolo-Aguilar et al. 2022). During cold response, the capacity for protein synthesis generally drops, apart from a brief spike in the production of certain proteins known as cold-shock proteins (CSPs). Furthermore, this phase of cold adaptation requires significant changes in gene expression. Although the synthesis rates of RNA, proteins, and lipids usually decrease, the production of a specific set of CSPs quickly increase (Giuliodori et al. 2023, Phadtare and Inouye 1999).
CSPs regulate gene expression through a series of molecular mechanisms spanning transcription, post-transcriptional modifications, and translation. These proteins modulate transcriptional activity by engaging directly with DNA's regulatory regions, such as promoters and enhancers, potentially acting as transcription factors or co-regulators. This interaction helps in assembling RNA polymerase and other essential components on specific genes. In bacteria, like Escherichia coli, CSPs bind to distinct DNA sequences, altering gene expression to manage stress responses, metabolism, and cold adaptation. CSPs also play a pivotal role in RNA stability under stress by binding to RNA, thus preventing its degradation and serving as RNA chaperones during cold shocks. This stabilization is crucial for the translation of proteins essential for cold adaptation, including molecular chaperones and metabolic enzymes. Furthermore, CSPs influence post-transcriptional processes, including RNA splicing, editing, and localization, by interacting with RNA-binding proteins and regulatory RNAs, thus managing transcript processing and placement. During cold stress, CSPs modulate protein synthesis by interacting with ribosomes and translation initiation factors, enhancing the production of mRNAs coding for cold-adaptive proteins and reducing the translation of non-essential proteins. They can also interact with RNA secondary structures, like RNA thermometers, to control the translation of cold-responsive genes by modulating ribosome access to the mRNA's start codon.
According to the studies, a rapid decrease in temperature (from 37°C to 10°C) triggered a 200-fold surge in the expression of cold shock protein A (CspA), and this response was not influenced by transcriptional activity (Gottesman 2018, Lindquist and Mertens 2018). Some CSPs play a crucial role in facilitating accurate and enhanced translation of messenger RNAs (mRNAs) specific to low temperatures (Al-Fageeh and Smales 2006, Dahlquist et al. 2015, Phadtare et al. 1999). The most thoroughly examined cold shock protein in Escherichia coli is known as CspA. This family consists of nine paralogues, of which seven—CspA, CspB, CspE, CspF, CspG, CspH, and CspI—are inducible by cold temperatures. In contrast, two of the paralogues, CspC and CspD, are expressed exclusively at 37°C (Goldstein 1990, Yamanaka and Inouye 1997, Zhang et al. 2018, Giuliodori et al. 2019). In the case of E. coli, the cold shock response is essential for cell survival and enables cells to resume growth at unfavorable low temperatures. This process involves the modulation of DNA replication, transcription, translation, stabilization of RNA, and assembly of ribosomes (Gualerzi et al. 2003). The evolution of CSPs exemplifies the remarkable diversity and flexibility of life in responding to and thriving in various environmental conditions.
Biophysical of Cold-Shock Proteins
The CSPs constitute a protein family known for substantially increasing expression levels in response to cold shock conditions. These proteins are often called Cold-inducible proteins (CIPs) or cold acclimation proteins (CAPs) due to their role in adapting cells to cold environments. CSPs are relatively small proteins, with an approximate size of 7.4 kD. What sets them apart is their unique ability to bind to nucleic acids, a characteristic that plays a crucial role in their functions. The hallmark of CSPs is the presence of a conserved structural domain called the cold shock domain (CSD). This domain is pivotal for the protein's function and is where nucleic acid-binding motifs, particularly RNP-1 and RNP-2, are located. RNP stands for Ribonucleoprotein, indicating the association of the protein with RNA molecules. The CSD is a structurally significant region that allows Csps to interact specifically with single-stranded RNA. The nucleic acid-binding motifs, RNP-1 and RNP-2, within the CSD are essential for mediating interactions with RNA molecules. These motifs contribute to the specificity of binding, allowing CSPs to recognize and associate with particular sequences or structures in RNA. The ability to bind nucleic acids, especially RNA, underscores the functional importance of CSPs in various cellular processes, particularly those related to the response to cold stress.
The structural characteristics of CSPs make them particularly intriguing subjects for detailed studies on conformational stability and folding kinetics. CSPs are notable for the presence of a fully formed hydrophobic core within their structure. Additionally, they lack disulfide bonds or cis-peptides, which are often associated with slower phases in the protein folding process. The absence of disulfide bonds and cis-peptides in CSPs is significant, as these structural elements can contribute to slower or more complex folding patterns in proteins. CSPs, by lacking these features, simplify the study of their folding kinetics and mechanism, providing researchers with clearer insights into the dynamics of their conformational changes. The findings suggest that CSPs have evolved to withstand extreme environmental conditions while maintaining a rapid and efficient folding mechanism.
The exploration of CSPs commenced with their identification in E. coli, notably the discovery of CspA (Fig. 2, Schindelin et al. 1994), response to a rapid drop in temperature during growth and regulates transcription for at least two genes. Following exposure to cold shock, CspA was found to constitute a substantial portion, approximately 15%, of the total protein synthesis in the cell. Initially thought to be exclusively involved in cold adaptation, subsequent observations unveiled a broader role for CspA.
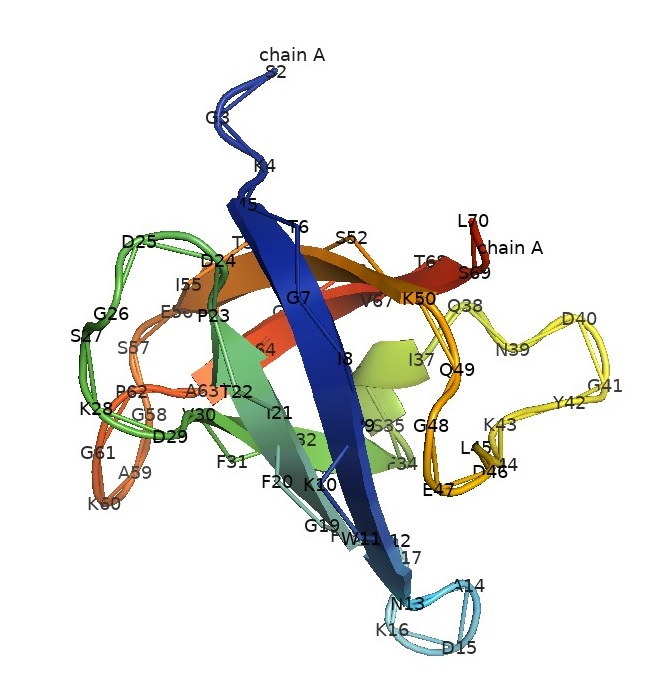
Fig. 2.
The crystal structure of cold shock protein A from Escherichia coli. CspA exhibits a significant resemblance to the nucleic acid-binding domain found in Y-box factors, which are eukaryotic proteins involved in regulating transcription and translation. The crystal structure of CspA, resolved at 2-A resolution, reveals a closed five-stranded beta-barrel formed by antiparallel beta-strands. While this structure resembles Bacillus subtilis' major cold shock protein, CspB, CspA does not form a dimer in the crystal. CspA's surface suggests interaction with single-stranded nucleic acids (Schindelin and Heinemann 1994).
It was discovered to be abundant not only during cold stress but also prominently present during the early exponential phase at a normal temperature of 37°C. However, the synthesis of CspA decreased as cells progressed to the late-exponential phase. A noteworthy experiment involved a quadruple knockout of CspA, CspB, CspG, and CspE in E. coli. This genetic manipulation rendered the bacteria incapable of dividing at low temperatures, showcasing the critical role of CSPs in the cellular response to cold conditions. The strong similarity among bacterial cold shock proteins, like E. coli CspA and B. subtilis CspB, establishes a shared structural framework for the cold shock domain (Schindelin et al. 1994). The cold sensitivity resulting from the quadruple knockout could be alleviated by the presence of any member of the CSP family, except for CspD. When CspD was overexpressed, it led to lethality by inhibiting DNA replication. These findings underscore the diverse functions and regulatory roles of CSPs in bacterial adaptation to cold environments. The CSPs are pivotal in responding to sudden cold shocks and normal growth phases, highlighting their versatility and significance in maintaining cellular homeostasis across varying temperature conditions.
Structural Feature of Cold Shock Proteins
Cold Shock Domain (CSD)
The CSD constitutes a crucial component of cold shock proteins, featuring a distinctive and intricate three-dimensional structure. Its compact and globular nature is paramount for ensuring the stability and functionality of the domain. This structural compactness confers resilience to the CSD and plays a pivotal role in facilitating its various biological functions. represents a simplified version of the oligonucleotide/oligosaccharide binding (OB) fold, distinct in that it lacks the α-helix component (Schindelin et al. 1992). CSDs are specialized motifs crucial for RNA and DNA metabolism activities, especially under cold stress conditions. Although simpler in structure when compared to the traditional OB fold, CSDs demonstrate a wide range of functions and play essential roles in the cold adaptation processes of various organisms. A distinctive feature of CSDs is their lack of the α-helix, a departure from the OB fold's typical structure. The α-helix, known for its coiled configuration created by hydrogen bonds among amino acids in the protein chain, is absent in CSDs. This absence indicates a structural simplification or variation within these domains, highlighting their unique adaptation for cold response.
The pioneer crystallized structure of CspB was reported in B. subtilis (Fig. 3). CspB is present as a compact molecule and is composed entirely of antiparallel β-sheet with connecting turns and loops. CspB contains two subdomains, the first consisting of strands β1 and β2 and a loop of 16 residues. The second consists of strands β3 and β4 (Schindelin et al. 1993).
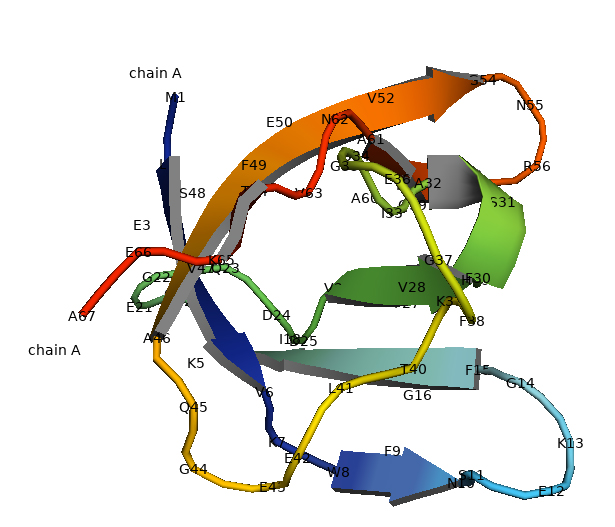
Fig. 3.
Crystal structure CspB of the Bacillus subtilis. The structure of CspB is characterized by a specific architectural pattern known as an antiparallel five-stranded β-barrel (Schindelin and Heinemann 1995).
The OB fold is exemplified by the distinctive five-stranded antiparallel β-barrel observed in CspB (Murzin 1993). In particular, the absence of the α-helix distinguishes it from the traditional OB fold. The OB-fold is a prevalent structural motif involved in nucleic acid binding, and the CSD, as a simplified version, retains its nucleic acid-binding capabilities. The crystallization of B. subtilis CspB marked a significant milestone, providing valuable insights into the structural organization of CSPs. The study on the crystal structure of CsdB of the B. subtilis is characterized by a specific architectural pattern known as an antiparallel five-stranded β-barrel. Within this barrel arrangement, there are three consecutive β-strands, and notably, the middle strand houses the RNP1 motif. The RNP1 motif is a conserved sequence pattern frequently found in RNA-binding proteins. This motif plays a crucial role in facilitating interactions between the protein and RNA molecules. Proteins containing the RNP1 motif often participate in various cellular processes related to RNA, such as binding, modification, or transport. Therefore, in the case of CspB, the presence of the RNP1 motif in the central β-strand of its β-barrel structure strongly suggests its potential involvement in interactions with RNA molecules. This implies that CspB may play a role in RNA-related processes, potentially influencing RNA binding, modification, or other functions within its structural framework (Schindelin et al. 1993). The determined structure, arising from two different crystal forms, not only illuminated the architecture of bacterial CSPs but also laid the foundation for understanding the conformation of the CSD in eukaryotic systems (Heinemann and Roske 2021).
β-strands arrangement
Embedded within the CSD are five β-strands arranged in an antiparallel orientation. The term “antiparallel” denotes the opposite directions of neighboring strands, a characteristic arrangement commonly observed in β-sheets. In β-strands, the term “antiparallel” denotes the alignment of adjacent strands in β-sheets in opposite directions, such that if one strand progresses from the N-terminus to the C-terminus, its neighboring strand will extend from the C-terminus to the N-terminus. This arrangement fosters a robust pattern of hydrogen bonding among the amino acids on adjacent strands. The integrity and robustness of β-sheet structures are significantly influenced by this antiparallel alignment. Compared to parallel β-sheets, antiparallel arrangements enable more direct and stronger hydrogen bonds between the amino acids, enhancing stability. Such antiparallel β-sheets are commonly found in proteins that demand high stability, like enzymes and structural proteins, due to their superior hydrogen bonding capabilities. This particular arrangement plays a crucial role in reinforcing the stability of the CSD, laying the groundwork for its diverse functions.
The assembly of these β-strands culminates in forming a barrel-like structure, often referred to as a β-barrel (Heinemann and Roske 2021). This architectural motif, prevalent in protein structures, imparts stability to the overall domain. Despite its compact size of approximately 70 amino acids, the CSD exhibits a fully formed hydrophobic core. This structural feature is noteworthy considering the domain's relatively small size. The hydrophobic core contributes to the stability and structural integrity of the CSD. Moreover, the CSD displays exposed aromatic residues on its basic protein surface. This particular arrangement strongly indicates a functional role in binding single-stranded nucleic acids. The exposed aromatic residues are positioned on a surface with basic properties, suggesting an affinity for interacting with nucleic acids, which often carry a negative charge. To validate the conjecture about its role in nucleic acid binding, experiments, such as gel-shift experiments, were conducted. In the case of the CSD, the gel-shift experiment specifically confirmed its binding capability to single-stranded DNA. The interaction between the CSD and ssDNA resulted in a detectable shift in gel electrophoresis, providing experimental evidence of the domain's ability to bind and potentially modulate the behavior of single-stranded nucleic acids.
The β-barrel structure serves as a structural scaffold, contributing not only to the stability of the CSD but also to its ability to interact with other molecules, particularly RNA. Surrounding the β-barrel are several α-helices, additional structural elements in proteins. These α-helices, through their spatial arrangement, further contribute to the overall stability and structural integrity of the CSD. The combined presence of β-strands and α-helix creates a sophisticated architecture essential for the proper functioning of the CSD.
The structural arrangement of β-strands and α-helix within the CSD is not arbitrary; it is a conserved motif observed across different bacterial species that possess cold shock proteins. This conservation underscores the functional importance of the CSD in the broader context of cold shock proteins. The consistent structure across diverse organisms implies a shared evolutionary pressure to maintain specific features critical for adaptation to cold environments. This conserved structural motif serves as a molecular fingerprint, highlighting the essential role of the CSD in facilitating the diverse functions of cold shock proteins across bacterial species.
RNA-Binding Site
The RNA-binding site within the CSD is a region characterized by specific amino acid residues that play a pivotal role in interacting with RNA molecules (Manival et al. 2001). This site is strategically positioned to enable the CSD to engage with the phosphate backbone and bases of single-stranded RNA. A key feature of the RNA-binding site is the presence of amino acids with properties conducive to forming interactions with RNA. These amino acids often have side chains that can establish hydrogen bonds or other favorable contacts with the functional groups on RNA. The specificity of these interactions is crucial for the CSD's ability to recognize and bind to RNA with high affinity. The electrostatic nature of the RNA-binding site is noteworthy. The positively charged surface of the CSD complements the negatively charged nature of RNA molecules. This positive charge arises from the amino acid residues within the RNA-binding site, creating an environment that facilitates electrostatic interactions. This electrostatic complementarity is a fundamental aspect of the molecular recognition process, allowing the CSD to effectively bind and interact with the negatively charged RNA. The electrostatic interactions between the positively charged CSD and the negatively charged RNA contribute to the stability of the RNA-protein complex. These interactions not only enable the initial binding of the CSD to RNA but also play a role in the overall structural integrity of the complex.
The positively charged surface acts as a molecular interface, fostering a strong and specific association with the RNA molecule. The RNA-binding capabilities of the CSD are central to its functional role within cold shock proteins. By interacting with RNA, the CSD participates in various cellular processes, including transcriptional and translational regulation, RNA metabolism, and RNA chaperone activity. The specific recognition and binding of RNA by the CSD exemplify the molecular adaptability of cold shock proteins in responding to environmental cues, such as sudden temperature downshifts.
Flexibility and functional versatility
The structure of CSPs, with particular emphasis on the CSD, is often characterized by a notable degree of flexibility. This inherent flexibility is a key attribute that contributes to the functional versatility of CSPs, enabling them to interact with a variety of RNA sequences and structures. The flexibility of the CSD is instrumental in facilitating the binding of CSPs to different RNA molecules. RNA sequences can exhibit diverse structures, and the flexibility of the CSD allows it to adapt to these variations. The dynamic nature of the protein structure enables it to accommodate different conformations of RNA, broadening the range of RNA sequences with which CSPs can interact. This adaptability is crucial for the proteins' role in diverse cellular processes, including transcriptional and translational regulation. One significant consequence of the flexibility observed in CSPs, especially within the CSD, is their ability to function as RNA chaperones. RNA chaperones are proteins that assist in the proper folding and structural rearrangement of RNA molecules. The dynamic nature of CSPs allows them to bind to unfolded or misfolded RNA structures and facilitate their correct folding into functional three-dimensional conformations.
CSPs, serving as RNA chaperones, play a crucial role in maintaining RNA integrity and functionality, particularly during cold shock. Their function as RNA chaperones highlights their importance in enabling cellular adaptation to temperature changes. In the face of cold stress, CSPs support RNA folding—a vital process for sustaining cellular activities—as cells endeavor to preserve correct RNA structures. Found in bacteria and various organisms, CSPs detect and attach to misfolded RNA, directing them toward proper folding routes. The process of RNA folding, indispensable for its function, is aided by CSPs through their binding interactions, guaranteeing that RNA molecules attain their functional shapes. This complex interaction emphasizes the role of CSPs in responding to environmental changes and ensuring cellular equilibrium under cold conditions.
Oligomeric structures formation
The cold shock proteins, have been observed to form oligomeric structures. This ability to associate and create oligomers varies among different CSPs, impacting their functional properties. The specific oligomeric state, whether dimers, trimers, or higher-order structures, can differ between these proteins. The formation of oligomeric structures is not just a quirk of protein behavior; it holds biological significance. In the case of CSPs, oligomerization is often linked to their functional activities. It's known that the arrangement of multiple subunits can influence the stability, substrate binding, and enzymatic activity of proteins. This oligomerization phenomenon is particularly relevant in the context of the RNA chaperone function of CSPs. The ability of CSPs to assist in the proper folding of RNA molecules is associated with their oligomeric state. Oligomerization may enhance the efficiency of CSPs in binding to RNA, potentially increasing their avidity for RNA substrates. The oligomerization of CSPs has structural consequences. It induces changes in the overall structure and dynamics of the proteins. This variability in oligomeric states, and the association of oligomerization with biological activities like RNA chaperone function, adds depth to our understanding of how CSPs operate in cellular processes, particularly in responding to environmental challenges such as cold stress.
Conclusion
The study of CSPs reveals a fascinating adaptation strategy employed by microorganisms to thrive in extreme cold environments. The structural characteristics of CSPs, particularly the CSD, showcase a unique and conserved three-dimensional architecture crucial for nucleic acid binding. CSPs’ versatility is evident in their ability to act as RNA chaperones, facilitating proper folding and structural rearrangement of RNA molecules. This comprehensive analysis contributes valuable insights into the biophysical properties of CSPs, shedding light on their structural features, RNA interactions, and functional adaptability. Understanding these molecular mechanisms not only expands our knowledge of cellular responses to cold stress but also provides a foundation for exploring broader implications in the context of microbial life in extreme environments. Ultimately, the study of CSPs adds a layer of complexity to our understanding of how microorganisms ingeniously adapt to challenging environmental conditions, opening avenues for further research and potential applications in biotechnology and synthetic biology.